Abstract
Background:
Alloimmunization is a frequent complication of red blood cell (RBC) transfusion in sickle cell disease (SCD), arising from disparities between the recipient and donor RBC antigens. This potentially life-threatening complication impacts the utilization of chronic transfusion, a disease-modifying treatment in SCD. Many centers across the country use dedicated donor programs that match SCD recipients to a small group of racially and phenotypically-matched RBC donors in order to minimize exposure to foreign RBC antigens. The pathogenesis of alloimmunization remains an area of active research. Emerging data suggests that a pro-inflammatory state in the recipient plays a role in alloantibody (alloAb) generation. Some SCD patients develop an alloAb after minimal exposure to donor RBC units, whereas others do not. In a subset of these latter patients, multiple exposures to disparate RBC units will not lead to alloAb formation. To further understand this phenomenon, we sought to identify patient-specific factors in SCD that are associated with a higher risk of alloimmunization.
Methods:
We retrospectively analyzed clinical, laboratory, and transfusion-related data for all pediatric and adult SCD patients treated at the University of Missouri between 2000-2017. Descriptive statistics were expressed for continuous variables. Two-tailed Student's t-tests and Chi-squared analyses were used to determine statistical significance.
Results:
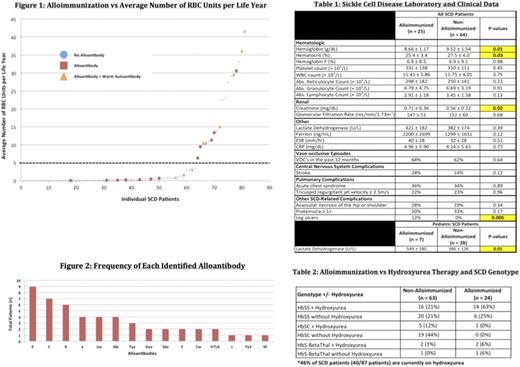
Of a total of 130 SCD patients identified, 89 patients (58 HbSS, 25 HbSC, and 6 HbSβ0/+ patients) had adequate transfusion, clinical, and laboratory data for inclusion in this study. The overall alloimmunization rate was 28%, with genotype-specific alloimmunization rates of 36% (HbSS), 4% (HbSC), and 50% (HbSβ0/+). Neither age nor gender significantly differed between the alloimmunized and non-alloimmunized groups.
Alloimmunized SCD patients received, on average, 4 times more lifetime RBC units as compared to the non-alloimmunized group (264 units vs. 65 units, respectively, p = 0.002). Among all SCD patients, individuals who received more than 5 units per year of life were more likely to become alloimmunized (61% vs. 16%, p = 0.02, Figure 1). The most common alloantibodies identified in our patients were C, E, and Kell (Figure 2). Warm autoantibodies were identified in 36% of all alloimmunized patients versus only 5% of non-alloimmunized patients (p = 0.0001).
Lastly, alloimmunization was associated with lower hemoglobin (8.66 ± 1.17 vs. 9.52 ± 1.54, p = 0.01) and higher creatinine (0.71 ± 0.36 vs. 0.56 ± 0.22, p = 0.02) among all SCD patients. In addition, higher LDH (549 ± 180 vs. 386 ± 127, p = 0.01) was observed in pediatric SCD patients who were alloimmunized. Both pediatric and adult SCD patients with the HbSS genotype demonstrated higher creatinine in the alloimmunized group (p = 0.03). An increased incidence of leg ulcers was found to be associated with alloimmunization (12% vs. 0%, p = 0.005, Table 1) amongst all of the clinical variables analyzed. Hydroxyurea therapy was not associated with a decreased prevalence of alloimmunization (Table 2).
Conclusions:
We identified an association between alloimmunization and higher LDH, lower hemoglobin, and higher creatinine in our SCD patient population. In addition, increased alloimmunization was seen in patients with a higher exposure to RBC units, both cumulatively and on a per life year basis. These data suggest that SCD patients with increased hemolysis or recipients of more than 5 RBC units per life year are associated with a higher risk of developing an alloAb. Larger studies will be required to define a causative relationship between this clinical SCD phenotype and a higher risk of alloimmunization.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal